Η ενημέρωση είναι στο χέρι σας - Πληροφορίες για τους ασθενείς
ΩΝΑΣΕΙΟ ΚΑΡΔΙΟΧΕΙΡΟΥΡΓΙΚΟ ΚΕΝΤΡΟ
Υπηρεσίες / Τομείς / Τμήματα
Ιατρική Υπηρεσία
Καλύπτει το πλήρες φάσμα διάγνωσης και θεραπείας όλων των παθήσεων της καρδιάς και στοχεύει στην παροχή εξατομικευμένων φροντίδων υγείας υψηλοτάτου επιπέδου, στη διεξαγωγή έρευνας και στην εκπαίδευση νέων ιατρών.
Νοσηλευτική Υπηρεσία
Διασφαλίζει ότι τα νοσηλευτικά τμήματα είναι στελεχωμένα βάσει διεθνών προδιαγραφών, που εγγυώνται την ασφάλεια και την υψηλού επιπέδου νοσηλευτική φροντίδα.
Διοικητική / Οικονομική / Πληροφορική Υπηρεσία & Αυτοτελή Γραφεία
Τις εξειδικευμένες υπηρεσίες του Ωνασείου υποστηρίζουν με το έργο τους πολλές ακόμα ειδικότητες που εντάσσονται στη Διοικ./Οικον./Πληροφ. Υπηρεσία & τα Αυτοτελή Γραφεία.
Τεχνική Υπηρεσία
Φροντίζει για την αγορά, τη συντήρηση, την ανανέωση και την αποδοτική λειτουργία του κτιριακού, ηλεκτρομηχανολογικού και βιοϊατρικού εξοπλισμού, την κάλυψη των ενεργειακών αναγκών του Νοσοκομείου και την τήρηση των περιβαλλοντικών απαιτήσεων λειτουργίας.
Ιατρική Υπηρεσία –Καρδιοχειρουργικός Τομέας
Εφαρμόζονται όλες οι προηγμένες θεραπευτικές μέθοδοι & επεμβάσεις, συμπεριλαμβανομένων και των υπηρεσιών σε νεογνά, βρέφη, παιδιά και ενήλικες με σύμπλοκες συγγενείς καρδιοπάθειες, ενώ λειτουργεί και το μοναδικό στη χώρα Πρόγραμμα Μεταμοσχεύσεων Καρδιάς.
Ιατρική Υπηρεσία –Καρδιολογικός Τομέας
Ο Καρδιολογικός Τομέας είναι πρωτοπόρος στην εφαρμογή νέων τεχνικών ενώ παράλληλα δραστηριοποιείται έντονα στην ιατρική εκπαίδευση και την ιατρική επιστημονική έρευνα.
Ιατρική Υπηρεσία –Αναισθησιολογικός Τομέας
Ο Αναισθησιολογικός Τομέας ανταποκρίνεται στις μεγάλες απαιτήσεις της εξειδίκευσης της Καρδιοαναισθησιολογίας και Παιδοαναισθησιολογίας, προσφέροντας υψηλής ποιότητας ιατρικές υπηρεσίες.
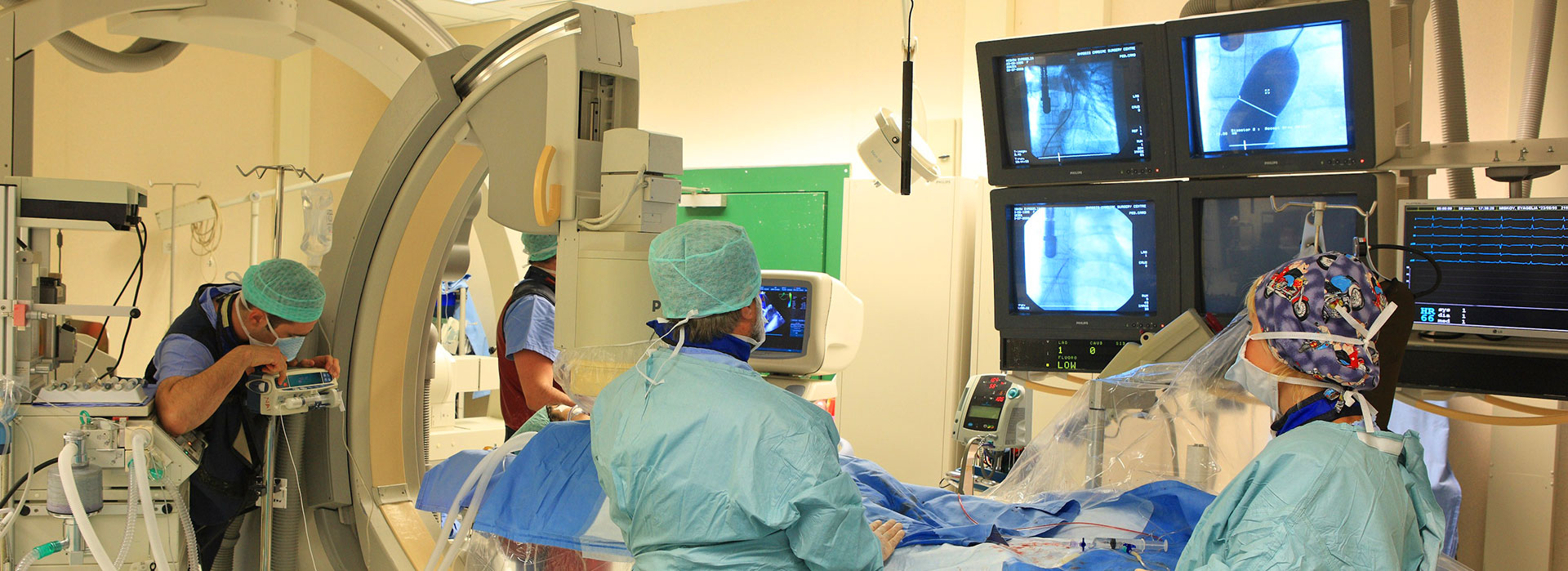
Ιατρική Υπηρεσία –Εργαστηριακός Τομέας
Ο Εργαστηριακός Τομέας είναι πλήρως εφοδιασμένος με τον πλέον σύγχρονο εξοπλισμό που καλύπτει όλες τις ανάγκες, από τις πιο απλές διαγνωστικές εξετάσεις μέχρι εξειδικευμένες και πρωτοποριακές εξετάσεις, σε εσωτερικούς και εξωτερικούς ασθενείς.
Ενημέρωση
Φυλλάδια
Υλικό για ΜΜΕ
Οι δημοσιογράφοι μπορείτε να επικοινωνείτε με το Γραφείο Διασφάλισης Ποιότητας – Δημοσίων Σχέσεων στα τηλέφωνα 210 94 93 188 – 210 94 93 000 και στην ηλεκτρονική διεύθυνση info@onasseio.gr.
Advanced Coronary Therapies 2024

Flyer Masterclass Athens

Ένωση Επιστημονικού Προσωπικού ΩΚΚ – 24th SYMPOSIUM ON ECHOCARDIOLOGY

Πρακτική Άσκηση στην Περιεγχειρητική Διοισοφάγειο / Διαθωρακινή Ηχοκαρδιογραφία (ΤΕΕ/ΤΤΕ)

H καλή καρδιά μετράει!

Cardiovascular Magnetic Resonance Imaging – Core Training (Level 1 SCMR Workshop)

Εργαλεία Προεγχειρητικής Αξιολόγησης Καρδιολογικών Παθήσεων Συνδυάζοντας Τεχνολογίες 3Δ Εκτύπωσης και Ψηφιακής Μοντελοποίησης Κλινικών Περιστατικών – 3D4KARDIA/Τ2ΕΔΚ-04012
ΒΑΣΙΚΕΣ ΑΡΧΕΣ ΕΦΑΡΜΟΓΗΣ ΜΕΜΒΡΑΝΗΣ ΟΞΥΓΟΝΩΣΗΣ-ECMO

Συνέδριο «Καρδιά σε Προχωρημένη Ανεπάρκεια: Σύγχρονες Θεραπευτικές Δράσεις – Προοπτικές»





















44 thoughts on “Front Page”
Comments are closed.